M03 - M04 The size of the atom and nucleus
Aim: To compare the size of an atom and its nucleus with some well known objects. |
The nucleus of an atom has no sharply defined boundaries and, although it can be considered spherical in form, care must be taken when speaking of its ‘radius’. The value observed depends on the method used to determine it. The density of the atomic material varies as a function of the distance from the centre, it being approximately constant up to a certain distance from the nucleus, but then decreasing gradually, becoming zero at the nuclear surface. The nuclear radius, R, can be defined as the distance from the centre to the point where the density has decreased to half its original value. These radii can be obtained experimentally by deflection experiments using fast neutrons. The results show that the nuclear radius is proportional to A1/3 where A is the mass number:

The volume of a nucleus is therefore approximately proportional to the number of particles
in the nucleus, protons and neutrons, collectively known as nucleons. This suggests that
the nucleons are found equidistant from one another, irrespective of the number of
particles and hence the volume of a nucleon in any nucleus is constant.
Another conclusion is that the density of all nuclear material is a constant.
The mass, M, of a nucleus with a mass number A is given approximately by:

and is independent of A. This density is approximately 1014 times
larger than everyday material and gives an idea of the compactness of the nucleons in a
nucleus. A typical density for commonly encountered materials is 103 (kg m-3)
which leads to the conclusion that, outside the nuclear material, normal objects must
largely consist of empty space.
For Na (11 protons, 12 neutrons) the nuclear radius is 3.7 x 10-15 m and the
atomic radius is 1.86 x 10-10m. The nucleus is 40,000 times smaller than the
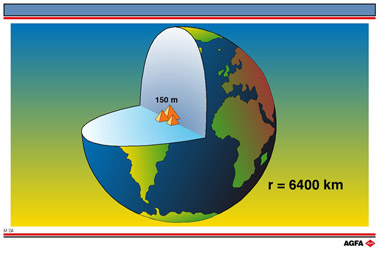
atom. The relative size of the nucleus compared with an atom is that of the height of the
Pyramid of Cheops (150m) to the radius of the earth (6,400,000m) (Illustration M4).
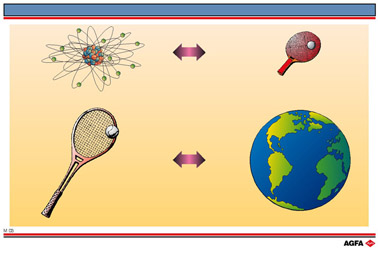
Alternatively, the size of an atom relative to a table tennis ball is equivalent to that
of a tennis ball relative to the earth. (Illustration M3).
Reference:
D. Halliday, R. Resnick and K. Krane, ‘Physics Vol II’,
‘J. Wiley & Sons, New York
J.T. Hewlis, R.C. Glass, D.J. Hughes, A.R. Meetham,
‘Encyclopaedic Dictionary of Physics’, Pergamon Press,
New-York (1975).